Abstract
Introduction: Sickle cell disease (SCD) vaso-occlusive pain episode (VOE) management often includes non-steroidal anti-inflammatory drugs (NSAIDs) and opioids, which can be inadequate and associated with significant side effects. Ketamine, a dissociative anesthetic, produces potent analgesic effects by blocking N-methyl-D-aspartate (NMDA) receptors, which impairs sensitization of spinal neurons to nociceptive stimuli. This mechanism allows ketamine to mitigate neuropathic pain and modulate opioid tolerance and opioid-induced hyperalgesias. Although only FDA-approved for use as an anesthetic agent, limited data suggest that low-dose ketamine is a safe and potentially effective adjunctive treatment for VOE pain.
Objectives: This study aimed to characterize ketamine use for VOE management in children with SCD.
Method: This retrospective cohort study summarizes a single-center experience regarding the use of ketamine for inpatient management of VOE in 156 admissions from 2014 to 2020 in which 44 unique children received ketamine.
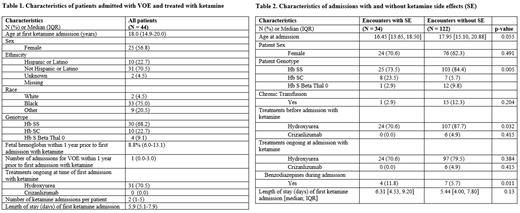
Results: Continuous low-dose ketamine infusion was most commonly prescribed to adolescents as an adjunct to opioids (median starting dose 2.0 mcg/kg/min; median maximum dose 3.0 mcg/kg/min). Ketamine was started a median of 13.7 hours after admission. Median ketamine infusion duration was 3 days (IQR 1.8-4.3). For admissions in which opioid was decreased during ketamine infusion, the median time from start of ketamine infusion to first opioid dose adjustment was 12.5 hours (IQR 3.25-36.6). In most encounters (n=119, 76.3%), ketamine infusion was discontinued prior to opioid PCA discontinuation. In 33 (21.2%) of encounters, the ketamine dose was decreased within 6 hours prior to discontinuation. The majority of encounters (79.3%) had a reduction in either PCA dose, continuous opioid infusion, or both while receiving ketamine. Low-dose ketamine infusion was well-tolerated overall, with side effects noted in 21.8% (n=34) of encounters. The most common side effects included dizziness (5.6%), hallucinations (5.1%), dissociation (2.6%), and sedation (1.9%). Nearly all side effects were reversible, resolving within 24 hours of dose reduction (n=11, 78.6%) or infusion discontinuation (n=21, 95.5%). There was no significant difference in patient sex or age at admission between groups with reported side effects compared to those without. There was a significant difference in hemoglobin genotype between groups (p=0.005). In both groups, most patients had hemoglobin SS. However, a higher percentage of encounters with side effects included patients with hemoglobin SC. Further, a higher percentage of patients in the no side effect group received hydroxyurea before admission (p=0.032) (Table 2). There were no reports of ketamine withdrawal. In 11 (7.1%) encounters, benzodiazepines were administered during ketamine infusion; 7 intravenously and 4 enterally. In most cases (n=5, 45.5%) the benzodiazepine was given for anxiety, in 2 cases (18.2%) for spasticity, 2 (18.2%) for spasm, 1 (9.1%) for agitation and 1 (9.1%) for pain. Benzodiazepines were administered within 12 hours after ketamine initiation in only 3 cases. Most patients who received ketamine went on to receive it again during a subsequent admission (72.1%, n=98).
Conclusion: Ketamine infusion should be considered when developing individualized pain plans for children with SCD, and it may be particularly beneficial for those who have a history of inadequate pain relief from opioids or dose-limiting opioid side effects. The variability of ketamine administration highlights the need for standardized protocols for ketamine use in VOE management. Further prospective study is needed to establish the efficacy of ketamine infusion and to determine optimal dosing, which may be patient-specific.
Disclosures
Heeney:FORMA Therapeutics: Consultancy; Oric Pharmaceuticals: Consultancy; Vertex/ Crisper Therapeutics: Consultancy; Bluebird Bio: Consultancy; Novartis: Consultancy. Archer:Global Blood Therapeutics: Research Funding; Haemonetics: Other: Stocks are provided to my husband as part of his compensation as an employee of the company; Novartis: Research Funding.
OffLabel Disclosure:
Ketamine is only FDA-approved for use as an anesthetic agent. However, limited data suggest that low-dose ketamine may be a safe and effective adjunctive treatment for vaso-occlusive pain.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal